It’s like the parable about the blindfolded men and the elephant—only instead of an elephant, it’s an enzyme.
For decades, researchers have groped at a family of proteins called Rafs. These proteins—including A-Raf, B-Raf and C-Raf—transmit signals that control proliferation, differentiation and survival in every cell in the body.
Raf proteins, especially B-Raf, are also well-known cancer drivers. Hence Raf’s full name: rapidly accelerated fibrosarcoma. Faulty control of their activity can cause melanoma; thyroid, colorectal, non-small cell lung and pediatric brain cancers; and other malignancies. Two FDA-approved drugs treat cancer by inhibiting B-Raf.
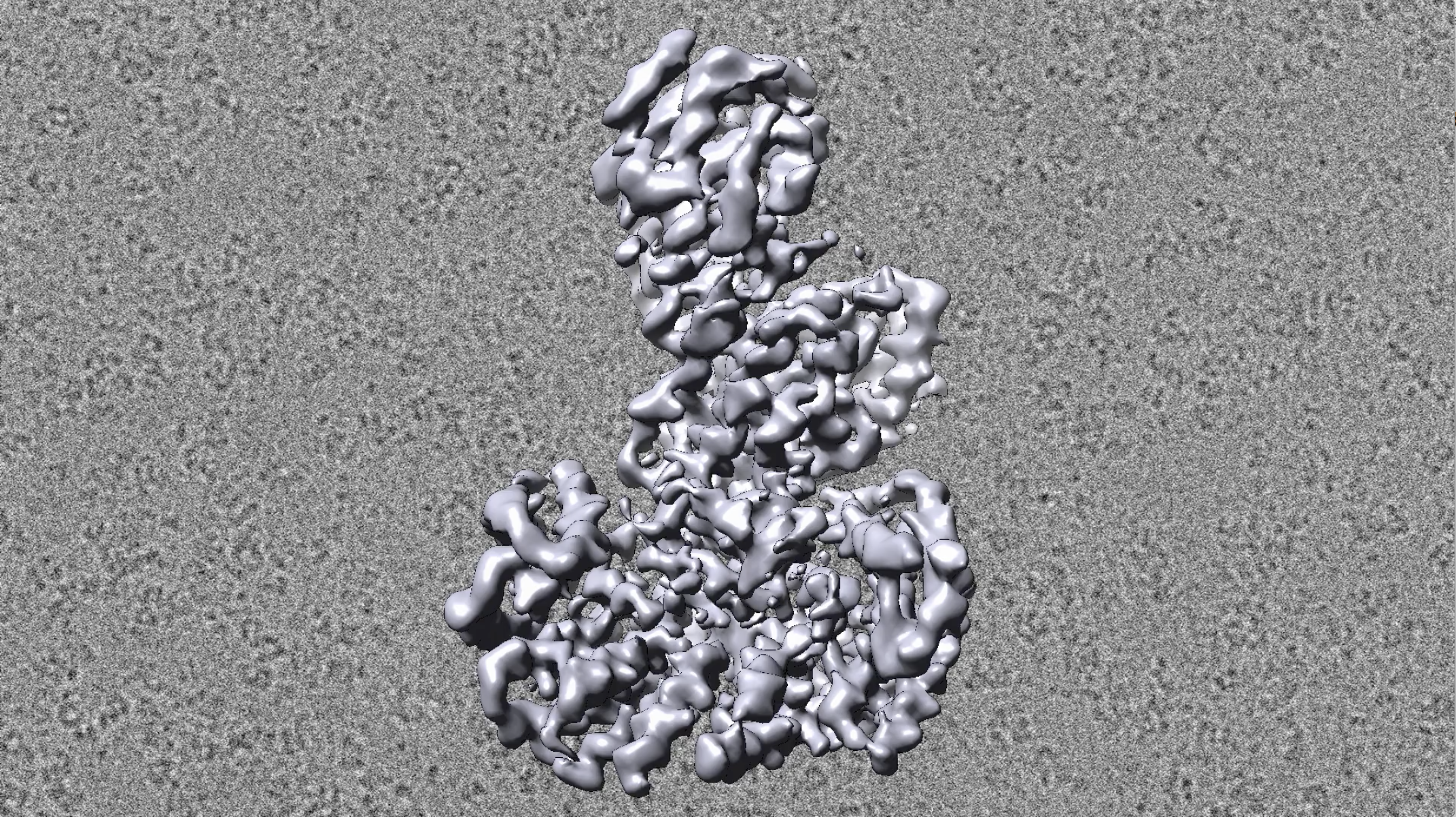
Given B-Raf’s critical roles in health and disease, scientists have been keen to understand its structure. They’ve used genetics, chemistry, biology and other scientific approaches, but they haven’t been able to piece together a complete picture of Raf.
“People had poked and prodded Raf for more than 30 years, but we could only see parts of it,” said Michael J. Eck, professor of biological chemistry and molecular pharmacology in the Blavatnik Institute at Harvard Medical School and Dana-Farber Cancer Institute.
Now, thanks to work by Eck and colleagues, researchers can see the whole enzyme.
As reported online this month in Nature, Eck’s team at last captured high-resolution images of B-Raf in its inactive or “off” state and in several active or “on” positions.
The findings clarify how B-Raf functions normally in the body as well as what happens when mutations alter its shape and lead to cancer.
Read the whole HMS article here.